Abstract
Background: Acute myeloid leukemia (AML) chimeric antigen receptor (CAR) T cell therapies are at early stages of testing in human clinical trials. We previously described the design of a CD33-specific dimerizing agent regulated immunoreceptor complex (DARIC33) that, in the presence of rapamycin (RAPA), switches from an "OFF" to "ON" state that activates T cells in response to tumor antigen. Here, we describe RAPA controlled activation and anti-AML activity of DARIC33 using human AML xenograft NSG mouse models and GMP-compliant T cell manufacturing methodologies. We find that low nanomolar whole blood concentrations of RAPA, below levels used for immunosuppression, are needed for DARIC33 to become active in vivo and exhibit potent CD33-specific anti-AML activity.
Methods: Clinical scale, DARIC33 and control cell products were manufactured by Seattle Children's Therapeutics (SC-DARIC33) by stimulating equal numbers of CD4+ and CD8+ T cells with anti-CD3/CD28 microbeads in closed gas-permeable culture vessels followed by lentiviral vector transduction and expansion in serum-free media supplemented with IL7, IL15 and IL21. The activation and anti-AML activity of SC-DARIC33 assayed in vitro by measuring cytokine production or lysis of chromium labeled target cells in the presence or absence of RAPA. NSG mice inoculated with either 1x10 6 luciferase expressing MV4-11 (CD33+) or 0.5x10 6 Raji cells expressing a huCD33 transgene (Raji.CD33, CD19+/CD33+) were treated with SC-DARIC33 and RAPA. Concentrations of RAPA in mouse blood were quantified by LC-MS/MS (Charles River).
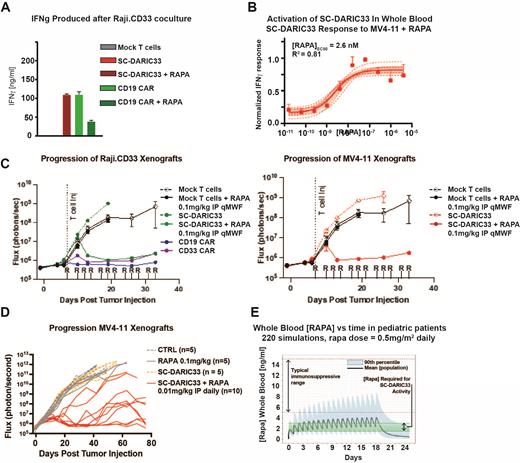
Results: Donor matched SC-DARIC33 and control CD19 CAR T cell products exhibited similar surface markers of engraftment fitness (CD62L+CD45RA+) and capacity for anti-tumor (CD27+CCR7+) effector function. Following coculture of SC-DARIC33 and Raji.CD33 cells without RAPA, concentrations of IL-2, TNF-α or IFN-γ were not increased in comparison to Raji.CD33 cells cocultured with mock T cell products. However, when exposed to 1 nM RAPA and Raji.CD33 targets, SC-DARIC33 produced cytokines in quantities similar to (Fig A). To determine the concentration of RAPA required to activate SC-DARIC33 in patients, DARIC33 T cells, CD33+ AML targets, and graded concentrations of RAPA were added to allogeneic human whole blood samples and plasma was recovered after 24 hours of incubation. RAPA addition increased IFN-g release with an apparent EC50 = 2.6nM (Fig B).
Anti-AML SC-DARIC33 activity and whole blood [RAPA WB] in tumor bearing mice were determined and compared to pediatric RAPA pharmacokinetic models to select appropriate clinical RAPA dose schedules. NSG mice inoculated with Raji.CD33 tumors treated with either 1x10 7 CD19 CAR T cells or 3x10 7 SC-DARIC33 T cells, followed by RAPA 0.1 mg/kg IP QOD, exhibited stringent control of tumor growth, demonstrating a 3:1 cell dose equivalency (Fig C). AML progression was also inhibited when NSG mice were inoculated with MV411 AML cells and subsequently treated with 10 7 SC-DARIC33 followed by 0.01 mg/kg RAPA QD (Fig D). Blood samples from tumor bearing mice obtained on day 15, 2 hours post RAPA administration, showed [RAPA WB] = 2.3 ± 1.3 ng/mL, overlapping with the in vitroand indicating very low concentrations of RAPA effectively modulate the "OFF-to-ON" state transition. Population PK models simulating various RAPA doses and schedules in pediatric patients found oral daily dosing of 0.5 mg/m 2 RAPA will achieve [RAPA WB] = 1-3 ng/mL in most patients (Fig E).
Conclusion: Evaluation of GMP cell products and RAPA PK demonstrate that very low doses of RAPA are sufficient to regulate SC-DARIC33. To establish safety of SC-DARIC33 in humans, an upcoming phase 1 trial clinical trial evaluating SC-DARIC33 in pediatric AML patients will test escalating cell doses followed by low-dose RAPA administration from post T cell infusion days 2-21 using a Bayesian optimal interval (BOIN) design. Peripheral blood samples will be monitored for CD33+ myeloid cell recovery after cessation of RAPA dosing. These data will establish safety and support the feasibility of SC-DARIC33 CAR T cells to be reversibly modulated in an "OFF-ON-OFF" fashion by intermittent low-dose RAPA administration.
Price: bluebird, bio: Current Employment. Zhang: bluebird, bio: Current Employment. Sundaram: bluebird, bio: Current Employment. Lewis: bluebird, bio: Current Employment. Bilic: C4 Therapeutics: Current Employment. Xia: bluebird, bio: Current Employment. Krostag: bluebird, bio: Ended employment in the past 24 months. So: bluebird, bio: Current Employment. Martin: bluebird, bio: Current Employment. Leung: bluebird, bio: Ended employment in the past 24 months. Astrakhan: bluebird, bio: Current Employment. Pogson: bluebird, bio: Current Employment. Jarjour: bluebird, bio: Current Employment. Jensen: bluebird, bio: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal